By Pierre Servan CEO, Principal Consultant, Factor Quality Inc.
Do I need Document Control?
 In my years of performing third party Quality Management System (QMS) audits, gap assessments and internal audits, a common question about document control people ask is:
In my years of performing third party Quality Management System (QMS) audits, gap assessments and internal audits, a common question about document control people ask is:
“Do I need to control this document?”
Over the years I’ve noticed Document Control has received “a bad reputation” in quality circles. It does not matter if it’s a procedure, work instruction, or form; One of these pesky documents is bound to be in the wrong place at the wrong time.
Recently, I have noticed that these document control issues are decreasing due to new tech solutions that help organizations manage documents. Nevertheless, the question persists, “to control or not control a document?”
 Sponsored Article
Sponsored Article
Are you looking for recommendations for Quality software? Factor Quality has the experience you need for options you can rely on. Contact us Today!
To Control or not Control a Document?
Without dwelling in the details of ISO 9001 requirements regarding document control and in the spirit of keeping it simple, the intent of the standard’s requirement is straightforward. The aim of the standard is once your organization determines the need for a document (i.e. means to convey critical information or template to collect data) then logic dictates that you want to make it available to those who need it and want to make sure the information is always accurate.
“Document Control is having a way to ensure that information remains relevant, up-to-date, accessible and aligned to the strategy”. – Pierre Survan, Factor Quality
ISO 9001 does not handcuff organizations in dictating specific required procedures. Each organization is free to decide what documents need to be created and controlled.
The expectation is that when you make the decision you ensure the document aligns with the nature of the business and any requirements that need to be met.
Thou shall not use the standard’s name in vain
The ISO gives general rules for document control, that when used appropriately, do help businesses.
At Factor Quality, we’ve heard statements about document control such as:
“This document cannot be used because it is not in an ISO format.”
“That document requires to be approved by two supervisors, a manager and the CEO per ISO requirements.”
“That document is missing a document #, what section of the standard it belongs to so that I know how to number it.”
“That document is only important to our department, so it does not need to be controlled.”
Did you know ISO provides a general requirement for organizations, it does not tell you how many approvers certain documents need to have?
Nor does it prescribe a document format or a document ID# (i.e. QAP-1001ab). These are misconceptions that have been circulating for a long time.
Let’s clear some misconceptions:
- ISO 9001 does not establish a minimum of approvals required,
- ISO 9001 it does not provide a format for documents nor does it require you to have a document number.
“In my opinion, the “bad rep” of document control has mostly been driven by the way companies have decided to control their documents and some lack of understanding of the standard, leaving many confused and somewhat irritated. All these requirements are self-imposed by each organization. The company defines the policy of how many approvers are needed for documents, what type of format is to be used and how to identify it.” – Pierre Survan, Factor Quality
Important Note: Stay Curious and Question Decision Making – If you don’t like current document control methods within your organization, ask the owners of document control why they consider the current method correct. And under no circumstance should you accept “Because ISO requires it,” as an answer.

How Do We Control Documents?
While ISO 9001 does not mandate specific formats, identifier or number of approvals, the documents created for the organization must meet a certain set of criteria to be considered as controlled effectively.
Remember –when you create a document you need to make sure the correct version is available to all in the business.
Requirements You Need to be Aware of with ISO 9001
Documents can be in any media
“Any Media” means document scan be written in paper, electronic, even video formats. The documents can be written, pictorials, flow charts, or a combination of these. Just remember it needs to make sense to your organization.
Documents Need to be Identified
There is no need to have document numbers unless you believe these are needed and are helpful to your business. A simple identifier is the title of the document and if this appears in the footer or header of each page, the document is indeed identified.
Documents Need to be Approved
Designate a person or group of people with the authority to determine suitability for your business. Ideally, that person is always aligned to the strategic direction of the business and understands the implications of such a document.
Documents Need to be Controlled
- Version Control: Documents must have an identifiable version visible throughout the document. This allows you to determine if the right version of the document is being used. The version can be alphanumeric or by date.
- Distribution Control: Documents must be made available and accessible for use. They need to be maintained in a manner so points of use can be readily updated when changes occur, that only authorized changes are made, and documents remain legible over time.
Keeping Document Control Effective
Over the years some of the most infamous controls deployed by overly careful document control administrators have been:
- Document Stamps: Stamps showing the document status such as: “Reference Only,” “Uncontrolled,” “Not a Controlled Document,” “Master Copy,” etc.
- Footer Controls: “Not valid if printed,” “Check system for latest version,” “Not valid after 24 Hours,” etc.
- Watermark Controls: Using watermark to notate “Draft”, “Controlled”, “Uncontrolled”, etc.
All these are methods of control but can be misunderstood by those using them. For example, could you have the correct stamp, footer, or watermark, but have no way to ensure that people do not change the document, even on accident? Can these controls show that the document approvals were adequate? Can a stamp prevent someone from receiving an outdated version of the process?
Do not assume that if the correct stamp, footer, or watermark is used, that is enough to demonstrate robust document controls.
Remember, these are just controls and as such, auditors will always check for effectiveness.
It doesn’t matter how big and bright the stamp, footer, or watermark is – when evaluating Document Control, auditors will consider valid the document the employee/operator points or shows.
What is the Most Common Document Control Issue When Employees are Asked: “What Document do you Use?”
“Employees proudly say, ’this one’, as they pull it out from their toolbox or desk drawer, and in most cases, these copies are out of date”. – Pierre Survan, Factor Quality
Summary of document control
Document Control’s purpose is to help the business document those items that are critical to its own functionality.
These documents should align with the strategy and help the business meet requirements in a consistent manner.
How much or how little you control these documents is a decision made by each company.
At Factor Quality, our best advice is to Keep it Simple! The well-being of the organization should always be put before the need of a group or department. Make sure every document is needed, because when a document is really needed it will be easy to control, maintain and keep relevant.
Document control structures can be reviewed, revamped and reset during the lifetime of a Quality Management System. Organizations should review their systems and ensure that they are leveraging existing and available resources to support their own documented systems. Factor Quality is here to guide you through this unpopular mythical creature called Document Control without Process Improvement Services. We have experience using several document control systems, from software solutions to self-contained systems, we can recommend the best simple, but effective solution for your business.
Let us help you set a new document control structure or revamp your current system to its most effective. Take advantage of our knowledge in the industry! This article was originally published here by Factor Quality and has been published on ISOUpdate with permission from Pierre Servan.
About the Author
Pierre Servan | CEO, Principal Consultant, Factor Quality Inc.
 Factor Quality was founded in 2011, with a vision to help fix quality issues, improve businesses, and help them get certified. Pierre never thought he would encounter such a rewarding industry with clients that appreciated his work, students that appreciated his words, partners that helped him and consultants/colleagues that appreciated him and what he had to say. Today, Factor Quality helps organizations take the next step in their quality journey and service the following certification: ISO 9001, ISO 14001, ISO 13485, ISO 16949, ISO 17025, ISO 45001, AS9100, AS9110 & AS9120. If you are interested in learning more about Factory Quality, visit them at www.factorquality.com/
Factor Quality was founded in 2011, with a vision to help fix quality issues, improve businesses, and help them get certified. Pierre never thought he would encounter such a rewarding industry with clients that appreciated his work, students that appreciated his words, partners that helped him and consultants/colleagues that appreciated him and what he had to say. Today, Factor Quality helps organizations take the next step in their quality journey and service the following certification: ISO 9001, ISO 14001, ISO 13485, ISO 16949, ISO 17025, ISO 45001, AS9100, AS9110 & AS9120. If you are interested in learning more about Factory Quality, visit them at www.factorquality.com/
 QAS International are ISO Consultants – developing, implementing and internally auditing a range of ISO Management Systems including ISO 9001 Quality Management Systems, ISO 45001 Safety Management Systems and ISO 14001 Environmental Management Systems. Our speciality is designing business management systems that don’t just tick boxes, they make real improvements. We engage with your employees to develop and implement ISO management systems tailored specifically for your organisation. We consider ourselves as part of your team and take pride in our ability to deliver bespoke, compliant (100% success rate) and cost-effective management systems that put organisations on the path to continual improvement and business excellence. Certified First Time, Every Time, Guaranteed!
QAS International are ISO Consultants – developing, implementing and internally auditing a range of ISO Management Systems including ISO 9001 Quality Management Systems, ISO 45001 Safety Management Systems and ISO 14001 Environmental Management Systems. Our speciality is designing business management systems that don’t just tick boxes, they make real improvements. We engage with your employees to develop and implement ISO management systems tailored specifically for your organisation. We consider ourselves as part of your team and take pride in our ability to deliver bespoke, compliant (100% success rate) and cost-effective management systems that put organisations on the path to continual improvement and business excellence. Certified First Time, Every Time, Guaranteed!










 Avital Koren is the Director of
Avital Koren is the Director of 
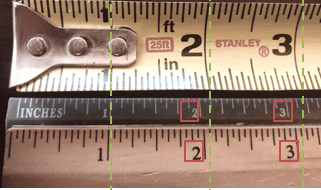 In this image, you will notice that at 1 inch, all rulers are measuring the same. But look at the 2- & 3-inch marks? They are all different. Which is the right measurement?
In this image, you will notice that at 1 inch, all rulers are measuring the same. But look at the 2- & 3-inch marks? They are all different. Which is the right measurement? Calibration stickers are the easiest way to identify your equipment and tools. The picture here is a snapshot of items you can find in google when searching for “calibration stickers”. You choose which best suits your organization and then start using it on all items that are calibrated. This is an easy way for everyone to know when to get the item calibrated.
Calibration stickers are the easiest way to identify your equipment and tools. The picture here is a snapshot of items you can find in google when searching for “calibration stickers”. You choose which best suits your organization and then start using it on all items that are calibrated. This is an easy way for everyone to know when to get the item calibrated.

 In my years of performing third party Quality Management System (QMS) audits, gap assessments and internal audits, a common question about document control people ask is:
In my years of performing third party Quality Management System (QMS) audits, gap assessments and internal audits, a common question about document control people ask is:
