The Benefits of Integrated Management Systems: Guest article from Steve Tyler, CEO & Founder of BusinessDocsOnline
Are your Business Management Systems still operating in Silos?
If so then you may want to think about adopting a more integrated approach…
Working in Silos?
There comes a point in the development of many organisations when they need to obtain some form of certification, and for the majority they will probably implement a management system for either Quality or Health & Safety.
There then follows a period of time where their requirements for certification will be covered with a single management system.
However, once an organisation grows to a point where it requires more than one management system, then that is the time for top management to step back and consider adopting a more integrated approach.
Yet too many organisations miss this opportunity and implement their management systems as stand-alone platforms. They then end up with individual management systems being used in silos.
For some organisations, working in silos may be the most suitable way to function, and there may be operational reasons why this approach works best for them.
But working in silos also has a downside…
Silo Mentality (as defined by the Business Dictionary):
 “a mind-set present when certain departments or sectors do not wish to share information with others in the same company. This type of mentality will reduce efficiency in the overall operation, reduce moral, and may contribute to the demise of a productive company culture.”
“a mind-set present when certain departments or sectors do not wish to share information with others in the same company. This type of mentality will reduce efficiency in the overall operation, reduce moral, and may contribute to the demise of a productive company culture.”
Whilst an integrated management system may not work for every organisation, for many the long-term benefits will far outweigh the short-term effort required to move forward.
So why not integrate your management systems and eliminate all the inefficiencies and duplication of activities that are part and parcel of having individual systems and working in silos?
But how easy is this to achieve?
The PDCA Cycle: – Plan – Do – Check – Act
With the latest release of ISO 9001:2015, this revised standard aims to further develop the “Risk Based Thinking” approach within an organisations. It also brings two other aspects into the management system arena that are going to redefine the future of management systems. One of these is Annex SL and the other is the PDCA cycle.
Lets come back to Annex SL later, and deal with the PDCA cycle first. Within ISO 9001:2015 this functions as follows:
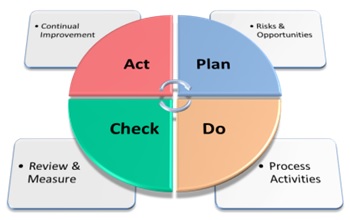
Plan
Top Management must assess the risks & opportunities that may impact on the organisation and carry out the planning required to ensure these risks do not affect the organisations ability to deliver its “desired outputs”. Exploiting any opportunities that have been identified must also be planned.
Do
Process activities must be carried out in such a way as to ensure they are aligned with the outputs of the planning processes.
Check
Top Management must review & measure the organisations performance against their objectives.
Act
Top Management must also plan & implement any actions that will deliver continual improvement.
Whilst the “desired outputs” of each organisation are quite unique, one way or another they all lead back to Customer Satisfaction. Once Customer Satisfaction can be monitored, it can be measured. And as the saying goes – “What gets measured gets done….”
So we can see how the PDCA cycle works for a Quality Management System, but this is really just the tip of the iceberg.
This PDCA cycle can now be applied to just about every other ISO standard, including Health & Safety [45001]*, Environmental [14001:2015] and Information Security Management [27001], and every system you implement can follow the same structure.
The net result here is that it is now possible to implement an integrated management system that combines Quality, Environmental, Health & Safety and Information Security.
But can they be that much more effective if they are integrated?
The Benefits of Integrated Management Systems
Once an organisation has decided to integrate their managem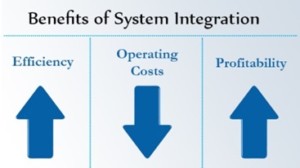 ent systems then it’s at this point they can start to see the real benefits.
ent systems then it’s at this point they can start to see the real benefits.
Organisations that have already implemented a single management system based around the PDCA cycle will find it up to 50% quicker when they come to implement their next management system.
The PDCA Cycle means it is possible to integrate your management systems into one platform, and organisations can now implement a single solution that controls all of the following:
- Risks & Opportunities for Product & Services
- Customer Requirements & Satisfaction
- Environmental Impacts
- Health & Safety Hazards
- Information Security Integrity
With this integrated approach, much of what is needed from the management team can now be done under one umbrella, and top management can now take a broader view of their organisation whilst undertaking the following activities:-
- Planning
- Assessments of Risk & Opportunities
- Internal Audits
- Management Reviews
- Continual Improvement
The end result is that:
- The organisation can now be managed using joined-up thinking.
- Auditing models can be revised to provide a much broader remit, but with fewer audits.
- KPI’s & SMART objectives can now become more aligned.
But just how well are all the different standards able to interact, and how easy is it to implement a single integrated platform across 2, 3 or 4 different management systems?
That’s where Annex SL comes in…
What is Annex SL?
 Annex SL is an ISO document that defines a high level structure [HSL] for the framework of a generic management system.
Annex SL is an ISO document that defines a high level structure [HSL] for the framework of a generic management system.
It was first published by ISO’s Technical Management Board (TMB) in 2012 and the recent release of ISO 9001:2015 has been revised to align with Annex SL.
Annex SL has arrived with a vengeance with the latest version of ISO 9001:2015, and is now here to stay.
In the future, all new ISO management system standards will adhere to the Annex SL framework and all current management system standards will migrate to it at their next revision.
As a result of the introduction of Annex SL, all ISO management system standards will become more consistent, and hence more compatible. They will share the same look and feel, having been built on a common foundation. The structure of all management systems will now include the following sections:
- Context of the Organisation
- Leadership
- Planning
- Support
- Operation
- Performance Evaluation
- Improvement
There are common core definitions too; the following words will have the same interpretations across all Annex SL standards:
- organisation
- interested party (preferred term)
- stakeholder (admitted term)
- requirement
- management system
- top management
- effectiveness
- policy
- objective
- risk
- competence
- documented information
- process
- performance
- outsource (verb)
- monitoring
- measurement
- audit
- conformity
- nonconformity
- correction
- corrective action
- continual improvement
Annex SL represents the beginning of the end of the conflicts, duplication, confusion and misunderstanding arising from subtly different requirements across the various management system standards.
Auditors now face the challenge of focusing their own, and their clients’, thinking on viewing organisations’ management systems holistically.
About BusinessDocsOnline












 “a mind-set present when certain departments or sectors do not wish to share information with others in the same company. This type of mentality will reduce efficiency in the overall operation, reduce moral, and may contribute to the demise of a productive company culture.”
“a mind-set present when certain departments or sectors do not wish to share information with others in the same company. This type of mentality will reduce efficiency in the overall operation, reduce moral, and may contribute to the demise of a productive company culture.”
 ent systems then it’s at this point they can start to see the real benefits.
ent systems then it’s at this point they can start to see the real benefits. Annex SL is an ISO document that defines a high level structure [HSL] for the framework of a generic management system.
Annex SL is an ISO document that defines a high level structure [HSL] for the framework of a generic management system.

